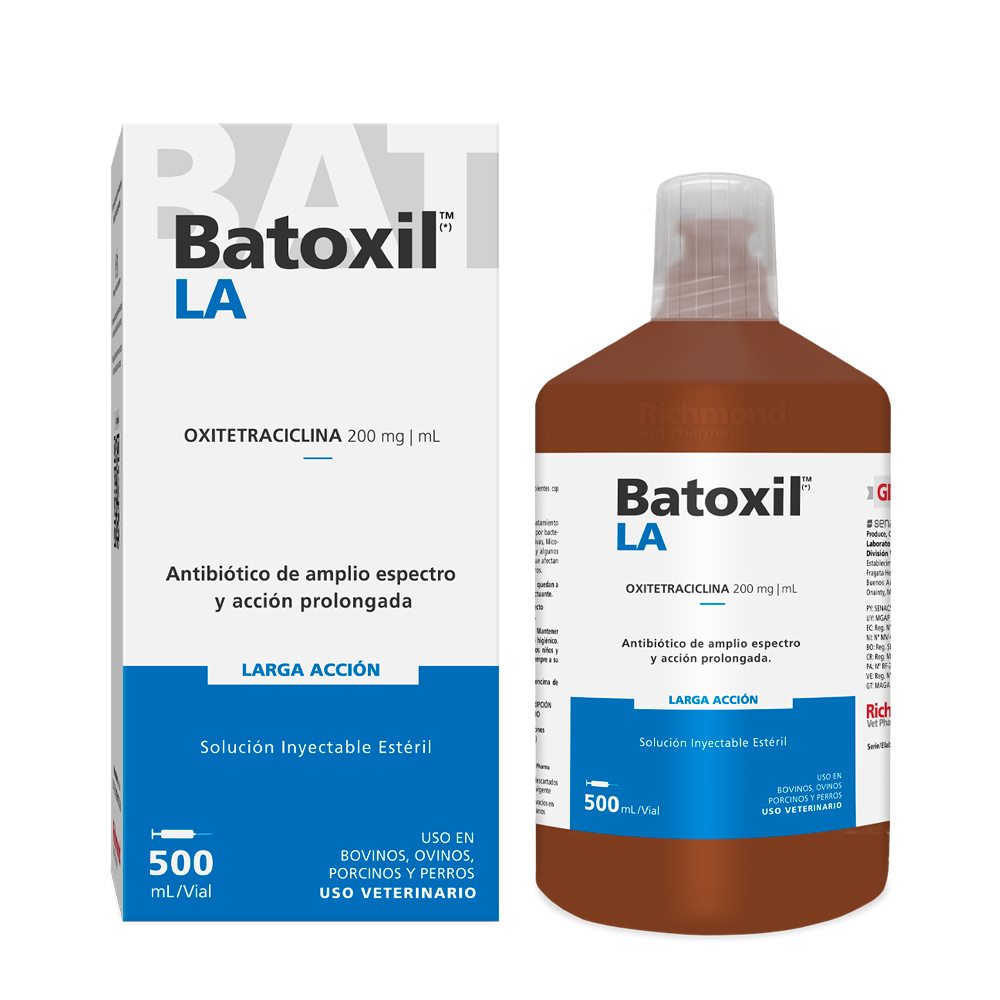
Batoxil LA
Registered Trademark of Richmond Veterinaria S.A.
- Oxytetracycline 20%.
- Long action
- Veterinary Use
Batoxil LA. Broad-spectrum, long-acting antibiotic.
Oxytetracycline 200 mg/mL. Ready-to-use sterile injectable solution.
Composition
Oxytetracycline Base 20.00 g, vehicles and formulation agents csp 100.00 mL
Batoxil LA is a sterile Oxytetracycline solution for injection (200 mg/mL). Bacteriostatic Antimicrobial, specially formulated with agents and vehicles that give its formulation a prolonged effect.
Batoxil LA achieves optimal concentrations in body fluids, serums and exudates. Approximately 23 hours in cattle and 18 hours in pigs, after the administration of the antibiotic, minimum inhibitory concentrations of the antibiotic are recorded, at the suggested dose. Serum antibiotic levels (0.2 mcg/ml) may be observed for 3 to 4 days after the application of Batoxil LA.
Oxytetracycline is derived from the metabolism of Streptomyces rimosus. It is an effective agent in the treatment of a wide spectrum of infections produced by Gram-positive and Gram-negative bacteria.
Batoxil LA is indicated as preventive and curative in infectious processes caused by Gram Positive, Gram Negative bacteria, Mycoplasmas, Chlamydia, Rickettsia and some fungi and pathogenic protozoa.
Its use is indicated in Bovine, Sheep, Pigs and dogs.
Indications
Because it is a broad-spectrum antibiotic, its use is suggested in Actinobacillosis (stick tongue produced by Actinobacillus lignieresii), Anaplasmosis, Bedsomiasis, Symptomatic anthrax, Infectious coryza, Diarrhea of young animals (Colibacillosis, Salmonellosis), Bacterial enteritis, Erysipelas, Transport fever (associated with Pasteurella sp and Haemophilus sp), nonspecific infections caused by Gram-positive and Gram-negative bacteria, postpartum (metritis) and post-surgical infections, Leptospirosis (Leptospira Pomona), septicemic mastitis, pneumonia and respiratory diseases, foot rot, keratoconjunctivitis (caused by Moraxella bovis), atrophic rhinitis and diphtheria caused by Fusobacterium necrophorum.
Medium Guidance Dosage
Cattle, Sheep, Pigs and Dogs
1 mL every 10 kg of weight
Deep intramuscular route. Do not apply more than 10 mL per point of application in cattle and no more than 5 mL in pigs, sheep and dogs.
Frequencies of application
When the severity of the illness requires it, the treatment should be repeated within 3 to 5 days after the first application.
Warnings
A slight inflammation with sensitivity reflexes may appear at the site of inoculation, an effect that disappears in a few days without the need for treatment.
In some hypersensitive animals, idiopathic anaphylactic reactions may occur, presumably due to prior sensitization with oil vaccine excipients. In this case, medication should be discontinued and Epinephrine 1/1000 – 1 mL / 50 kg weight should be applied by slow IV.
Restrictions
In animals intended for human consumption, treatment must be suspended 28 days before slaughter.
Do not administer in animals producing milk for human consumption or its industrialization.
Do not use the product after 28 days from opening.
Precautions
- Do not mix in the same syringe, cannula or container with any other substance not related to the product (other antibiotics, anti-inflammatories, anesthetics).
- Do not ingest. Keep out of reach of children and pets.
- Check the integrity of the seals before use.
- Keep the indications of asepsis and antisepsis before and during the application of the product.
- Do not administer by intravenous route.
- Store between 15 and 30 ºC, away from direct sunlight and in a hygienic place.
- Do not freeze.
Suggestions
It is suggested that the animals to be treated do not suffer from stress conditions before or after administration (prolonged confinement, mistreatment, exposure to high environmental temperatures for long periods of time and/or animals in a state of severe malnutrition).
If after the first application no remission of the initial signs or symptoms is observed, it is recommended to reevaluate the diagnosis or therapeutic.
Do not combine this antibiotic with bactericidal agents such as penicillin derivatives.
Always consult your Veterinary Doctor.
Recyclable packaging.
Destroy after use.
Do not contaminate the environment.
SALE UNDER PRESCRIPTION OF THE VETERINARIAN
Batoxil LA. Multi-dose vials
Containing 500 mL, 250 mL and
100 mL of Sterile Injectable Solution
Related products
Attention schedule:
Mon a Fri: 08:00-17:00
Where we are
Mail:
Fragata Heroína 4988 (B1615ICH)
Grand Bourg, Buenos Aires, Argentina.
Contact
Phone:
0810-333-7424
Correo electrónico:
info@richmondvet.com.ar