MIDAZOLAM
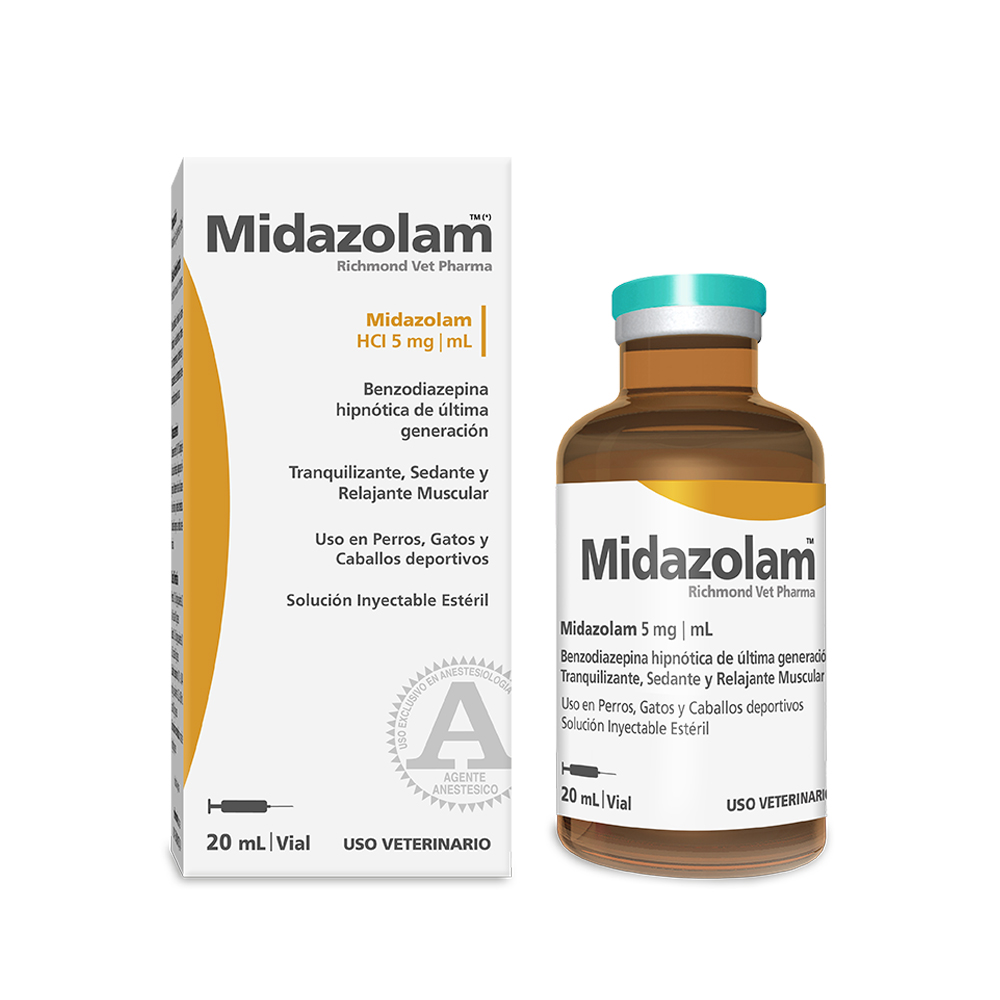
Midazolam TM
Midazolam Richmond Vet Pharma– Short-action benzodiazepine. Hypnotic, anxiolytic, sedative and muscle relaxant, for sport horses, dogs and cats. Ready to use sterile injectable solution.
5 mg/ml.
Veterinary use
Midazolam is an agent that derives from benzodiazepines; it can be used as anxiolytic, sedative, and hypnotic, anticonvulsive and muscle relaxant. It can be used alone or combined with analgesic, parasympathetic and anesthetics agents according to different protocols.
Midazolam is more efficient than Diazepam and its toxicity gets reduced to half. It has unique solubility characteristics; it is hydro-soluble in its original formulation stage and liposoluble at body pH level, which gives it speed of action after injection.
Midazolam is an hypnotic benzodiazepine of quick action; its effects lasts 2-6 hours. Provides a shorter induction time to anesthesia, allowing to reduce the doses of induction and maintenance agents.
Midazolam gives the patient a quiet awakening.
Composition
Midazolam 0.5 g; Excipients q.s.f. 100 mL
Pharmakodynamics
Midazolam acts selectively on the polysinaptic neural pathways mediated by GABA (Gamma AminoButyric Acid) or glicine.
Midazolam acts over all the CNS and almost exclusively its inhibitory action is pre-synaptic.
This pre-synaptic inhibitory action has the same power than Diazepam, but Midazolam’s efficiency is greater.
Midazolam acts mainly on ARAS (Ascending Reticular Activating System) and its action on this center is responsible for its anxiolytic, tranquillizing, hypnotic and calming effects.
The hypnotic effect of Midazolam is given by the depression of the cortical centers, and the muscle relaxation by the depression over the spinal motor centers.
Pharmacokinetics
Absortion
Following intramuscular injection, Midazolam is rapidly and nearly completely (91%) absorbed. Midazolam is also well absorbed after oral administration. But due to its rapid first-pass effect, its bioavailability ranges from 31 to 72%. The onset of action following IV administration is very rapid due to the high lipophilicity of the agent.
The drug is highly protein bound (94 – 97%) and rapidly crosses the blood-brain barrier.
Metabolism
Midazolam is primarily metabolized in the liver and gut by oxidation of the methyl group to its pharmacologic active metabolite, 1-hydroxy-midazolam and 4-hydroxy-midazolam. This metabolite is conjugated with glucoronic acid.
All metabolites are active prior to conjugation but their potency is infinitely less than the original compound.
This difference in potency cannot be explained by a lower affinity of the metabolites for the receptors, since it is similar to that of the original compound, but by a low penetration in the brain tissue.
Elimination
In dogs, half life of stage “B” (time required for excretion from peripheral compartment) of Midazolam is 1.1 hour. It’s excreted (oxidized and conjugated metabolites) exclusively in urine within 1- 1.5 hours.
Clearence in dogs is 29 ± 3 cm3/Kg/min.
Serum half-life and duration of activity of Midazolam is considerably shorter than that of Diazepam.
Compatibility
Midazolam can be dissolved in 0.9%solution of sodium chloride, glucose 5%, Ringer Lactate and Hartmann in a proportion of 15 mg of Midazolam every 100 – 1,000 ml solution to infuse. These prepared solutions are kept physically and chemically stable for about 24 hors, at room temperature, or for 3 days at 5ºC.
Midazolam is compatible with the following products: atropine sulfate, glycopirrolate, ketamine, morphine, fentanil and nalbuphine.
The compatibility depends on many factors such as pH, concentration, temperature, diluents.
Midazolam precipitates in sodium bicarbonate solutions.
Indications
Midazolam has a quick, intense and short sedative and hypno-inducing action,. It also has anxiolytic, anticonvulsive, tranquillizing and muscle relaxant properties.
- As part of preanaesthetic medication it can be used alone or in combination with analgesics, parasympatholytics (atropine), etc.
- It can be combined with ketamine or xylazine for minor surgery and diagnostic procedures.
- It can be combined with ketamine, tiopental and propofol to be used as an induction agent of anesthesia.
- It can be combined with acepromacine or ketamine during minor surgeries in cats.
- In dogs with cardiac disease.
- In thorax surgery in dogs.
- In intraocular surgery in horses.
- Muscular contractures: in patients with post-traumatic sympathetic dystrophy.
If Midazolam is compared with induction agents such as thiobarbiturics (thiopental), it has a minor cardiopulmonary depressor effect; it is hydrosoluble, it can be mixed with other agents, and it does not accumulate in the organism after repeated doses.
- Dosage and Application (Table 1)
Midazolam maximum dose – 1 mg/kg.
Hypnotic dose – the suggested dosage must be duplicated to produce a hypnotic effect.
Its intramuscular application is very well tolerated.
At this dosage and using Midazolam for premedication, barbiturates should be reduced in dose for induction.
- Minor surgery and diagnostic procedures (Table 2)
- Anesthesia induction: these protocols may be complemented with local infiltration anesthesia for minor surgery procedures or when epidural or regional anesthesia is used.
Midazolam / Ketamina
Midazolam 0.28 mg/kg IV equivalent to 0.056 ml/kg + Ketamine 5.6 mg/kg IV.
Midazolam / Tiopental
Midazolam 0.28 mg/kg IV equivalent to 0.056 ml/kg + Tiopental 3 to 8 mg/kg IV.
Midazolam / Propofol
Midazolam 0.28 mg/kg IV equivalent to 0.056 ml/kg + Propofol 2 mg/kg slow IV.
Apply additional doses of Propofol 0.5 mg/kg every 60 seconds until palpebral reflex disappears.
- Anesthesia induction in critical patients:
Midazolam / Propofol
Midazolam 0.28 mg/kg IV+ Propofol 1 mg/kg slow IV.
Apply additional doses of Propofol 0.5 mg/kg every 60 seconds until palpebral reflex disappears.
- Ambulatory surgery in cats:
Protocol A:
Acepromacina 0.01 mg/kg IM
Midazolam 1 mg/kg IM (0.2 ml/kg)
Ketamine 20 mg/kg IM
Fentanyl 0.02 mg/kg IM
Latency: 4.5 ± 0.7 min.
Analgesia: 50 ± 6 min.
Head movements: 54 ± 9 min.
Recuperation: 83 ± 13 min.
Protocol B:
Acepromazine 0.01 mg/kg IM
Midazolam 1 mg/kg IM (0.2 ml/kg)
Ketamine 20 mg/kg IM.
Latency: 4 ± 1 min.
Analgesia: 54 ± 12 min.
Head movements: 57 ±9 min.
Recuperation: 85 ±18 min.
- Dogs with cardiac disease:
Hypertrophic cardiomyopathy / Dilated cardiomyopathy
Premedication:
Midazolam 0.1 mg/kg IM (0.02 ml/kg)
Nalbuphine 0.4 mg/kg IM
Induction:
Tiopental 3-8 mg/kg IV or Propofol 1-2 mg/kg IV with supplementary doses of 0.5 mg/kg every 60 seconds.
Maintenance: Isoflurane 0.5-1.5% + Oxygen.
- Thoracic surgery in dogs
Continuous infusion:
Midazolam 0.55 ug/kg min + fentanil 0.7 ug/kg min.
- Intraocular surgery in horses:
Premedication: Romifidine 0.08 mg/kg IV.
Induction: Guaiacol glyceryl ether 100 mg/kg IV + Midazolam 0.1 mg/kg IV + Ketamine 1 mg/kg IV.
Maintenance: Halotane + Oxygen.
- Muscular contractures:
Post-traumatic sympathetic syndrome patients.
Painful syndromes as consequence of discopathies.
Midazolam can be used by IM route in combination with AINES.
The application interval for repeated treatments in indicated species is 2-6 hours.
- Contraindications and Limitations of use
Not for use in patients with hypersensitivity to the drug, serious hepatic or renal insufficiency, pregnant females and old or weakened patients.
Patients with congestive heart failure can eliminate the drug more slowly.
Midazolam should be carefully applied in patients under coma, shock or important respiratory depression.
- Pharmacological Interactions
Midazolam can enhance the central depressive effect if it is simultaneously applied with antipsychotics, hypnotics, anxiolytics, anti-depressives, analgesics, narcotics, antiepileptics, anesthetics, sedatives and antihistaminics.
There is a potentially significant interaction between Midazolam and the compounds that inhibit some hepatic enzymes (cytochrome P 450 III A). These compounds modify pharmacokinetics of midazolam and can induced prolonged sedation. This reaction occurs with cimetidine, erythromycin, diltiazem, verapamil, ketoconazole and itraconazole. Consequently, the patients who receive the cited compounds, and others that inhibit the P450 III A together with Midazolam must be carefully controlled during the first hours after administration of Midazolam (studies have shown that ranitidine does not influence the pharmacokinetics of Midazolam parenterally).
Interaction with many medicines which inhibits Midazolam hydroxylation (cyclosporine, amiodorone, neuroleptics) is possible.
- Precautions
Midazolam enhances the effect of barbiturates (Phenobarbital) and neuroleptics (chlorpromazine).
When administering midazolam associated with potent analgesics (tramadol, morphine), the latter should initially be applied, given that the dose of Midazolam based on its sedative effects, will be more safely regulated against sedation caused by the analgesic used.
Midazolam causes respiratory depression; its seriousness depends on the speed of administration, which will never be less than 30 seconds, otherwise an apnea phenomenon will be observed.
Midazolam should only be used if adequate resuscitation devices are available.
Store between 15-30ºC, protected from direct sun light, in dry and clean place.
Do not swallow. Keep out of reach of children and domestic animals.
The containers used must be discarded according to current legislation.
Sale under archived veterinary prescription.
11. Presentation – Vial per 20 ml.
Related products
Attention schedule:
Mon a Fri: 08:00-17:00
Where we are
Mail:
Fragata Heroína 4988 (B1615ICH)
Grand Bourg, Buenos Aires, Argentina.
Contact
Phone:
0810-333-7424
Correo electrónico:
info@richmondvet.com.ar